Lithium-Ion (Li-Ion) Batteries: The Backbone of Modern Energy Storage
As energy demands soar and portable electronics become increasingly vital to daily life, lithium-ion (Li-ion) batteries have emerged as the cornerstone of contemporary energy storage. Their widespread use spans smartphones, laptops, electric vehicles, and renewable energy systems—testament to their versatility, performance, and reliability.
Li-ion batteries operate on the principle of lithium-ion intercalation, typically using a graphite anode and a lithium metal oxide cathode. This configuration offers high energy density, long cycle life, and efficient charge-discharge behavior. Elements like cobalt and nickel in the cathode enhance thermal stability and storage capacity.
Despite their success, Li-ion batteries face challenges including safety concerns, resource scarcity, and environmental impact. Advances in solid-state electrolytes, battery recycling, and next-generation cathodes are paving the way for improved performance and sustainability, ensuring Li-ion technology remains at the forefront of energy innovation.
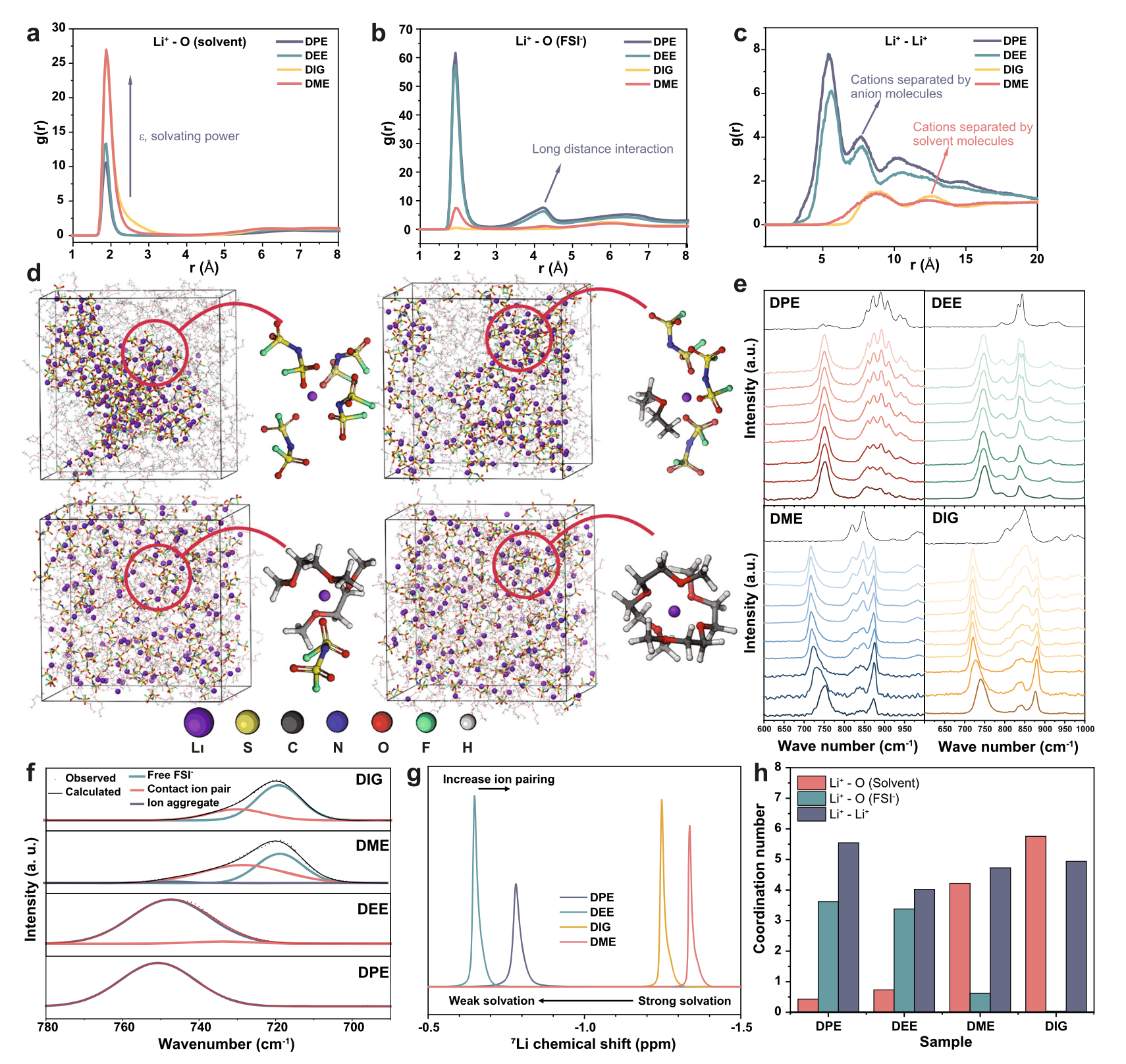
We demonstrate that in a non-polar dipropyl ether (DPE)-based electrolyte solution with lithium bis( uorosulfonyl) imide salt, the decomposition order of solvated species can be adjusted to promote the Li+/salt-derived anion clusters decomposition over free ether solvent molecules. This selective mechanism favors the formation of a robust cathode electrolyte interphase (CEI) and a solvent-deficient electric double-layer structure at the positive electrode interface.