Objectives
The development of powerful mobile devices, electrically driven cars, and renewable intermittent energy production fuels the ever-increasing demand for higher power and energy density in electrochemical energy storage devices.
One proposed way to achieve this goal is the creation of a device which uses materials from batteries and supercapacitors, called a hybrid energy storage device.
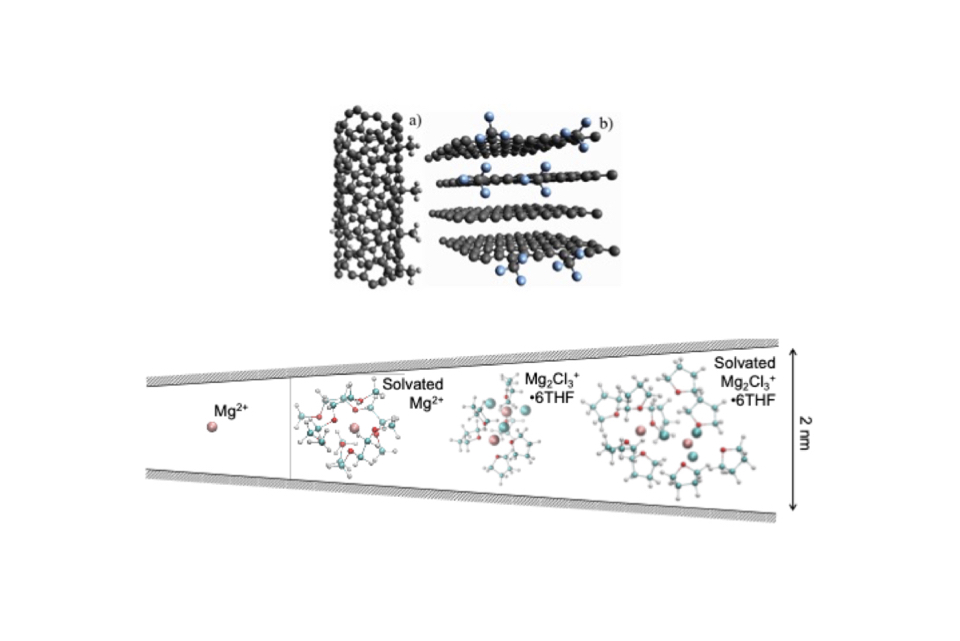
This project focuses on the computational screening of ionic liquid electrolyte for multivalent hybrid ion capacitors with the goal of being able to discover promising candidates for use in such a device.
In addition to running bulk calculations to elucidate the solvent structure and calculate properties such as electrochemical window, viscosity, and diffusivity, we are considering electrolyte confined in different nanoporous carbon electrodes.