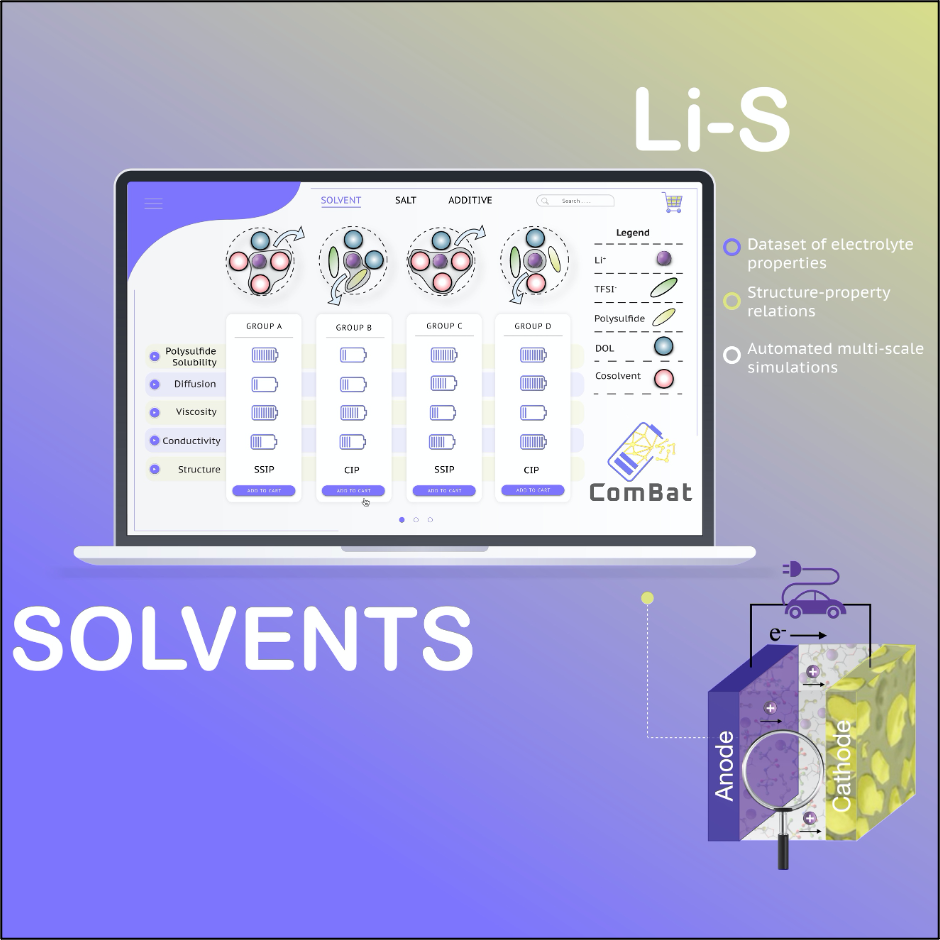
Lithium-Sulfur (Li-S) Batteries: Next-Generation Energy Storage Potential
Lithium-Sulfur (Li-S) batteries are one of the most promising candidates for next-generation batteries due to their higher theoretical energy density and lower cost compared to state-of-the-art Li-ion batteries. However, commercialization of Li-S batteries is hindered by their poor cycling performance and capacity retention caused by the formation and dissolution of lithium polysulfides (LiPSs) during discharging/charging in the electrolyte, the lifeblood of the battery.
More specifically, continuous diffusion of LiPSs towards Li metal anode and their nucleation and sluggish kinetics on the host carbon materials at the cathode result in loss of the active material. Hence, tailoring the atomistic interactions between LiPSs, salt anion, and solvent, in addition to the functional properties of porous carbon materials is critical in controlling deleterious side reactions, designing optimal electrolytes, and improving the performance and longevity of Li-S batteries.
Predicting and understanding the physicochemical behavior of bulk electrolytes and electrode-electrolyte interfaces is achieved via a multi-scale-data-driven approach that combines density functional theory (DFT) and molecular dynamics (MD) simulations.
The project is also done in close contact with experimentalists from PNNL, resulting in a better interpretation of experimental results and an enhanced predictive power of the next iteration of simulations.
The result is a database consisting of a large number of materials with optimized parameters to be used in electrolytes that achieve all the performance metrics for a given battery application.