Monovalent Batteries: Simplifying Ion Transport for Next-Gen Energy Storage
As energy storage systems evolve to meet global sustainability and performance demands, monovalent-ion batteries are gaining traction as a promising pathway. These systems rely on the movement of single-charged ions—such as lithium (Li⁺), sodium (Na⁺), or potassium (K⁺)—to deliver efficient, reversible electrochemical reactions with simplified transport dynamics.
Monovalent batteries offer distinct advantages due to their faster ion diffusion and lower charge-transfer resistance compared to multivalent systems. Their relatively straightforward chemistry enables high-rate capability and design flexibility, making them well-suited for both portable electronics and large-scale grid storage. Moreover, monovalent ions are generally more compatible with a wide range of electrode materials and electrolytes, facilitating rapid innovation across different chemistries.
Despite their appeal, monovalent batteries must overcome limitations in energy density and resource availability—especially in the case of lithium. Sodium and potassium-based variants offer earth-abundant alternatives, though they often require tailored host materials to accommodate larger ionic radii.
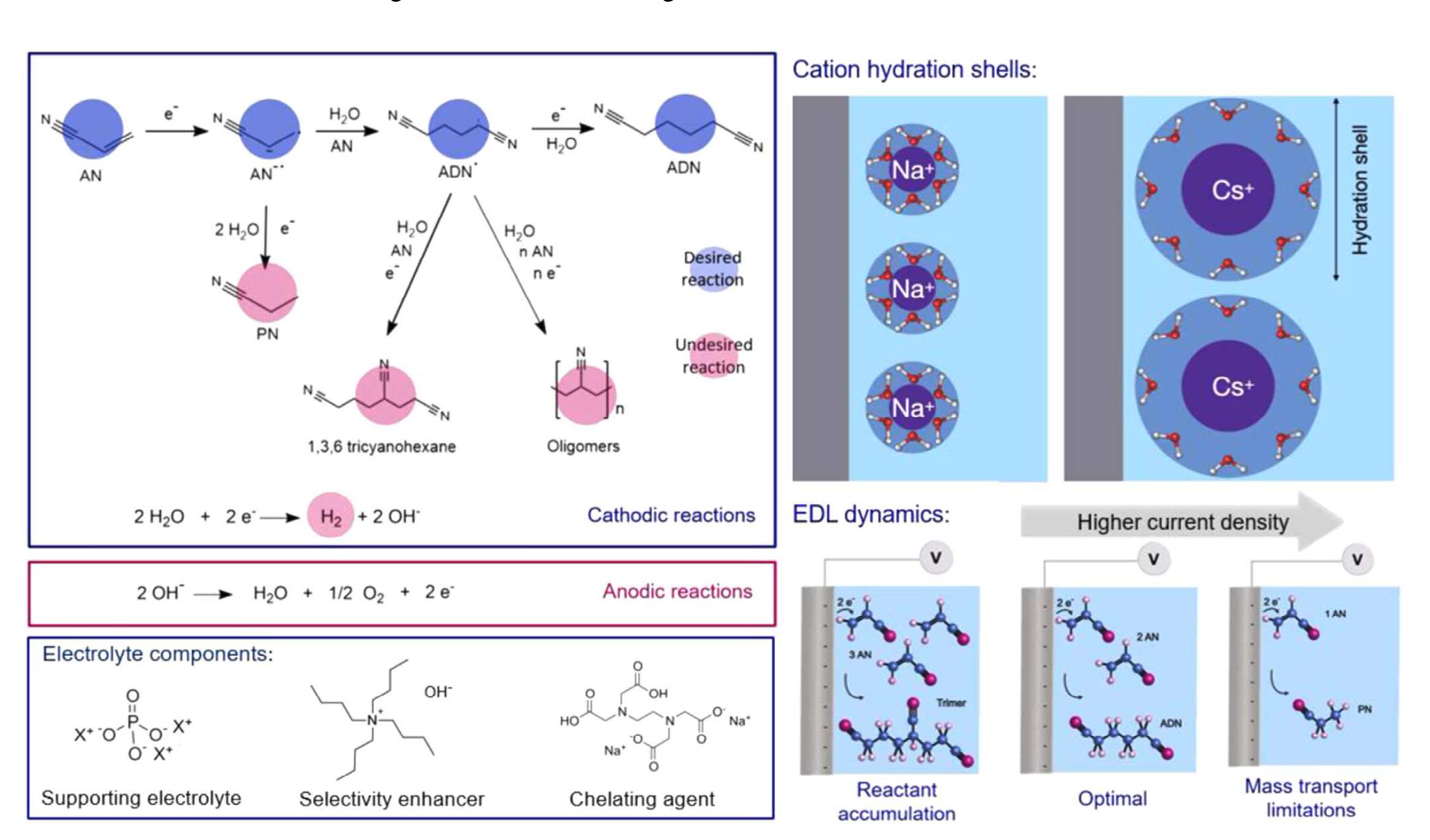
Our study elucidates the effect of electrolyte ions on reaction selectivity in the context of the electrohydrodimerization of acrylonitrile to adiponitrile. The size of supporting alkali cations has a profound effect on reaction overpotential and product distribution.